Intravenous Contrast and Renal Impairment
IODINATED CONTRAST AND IMAGING PATIENTS WITH RENAL IMPAIRMENT
Iodinated contrast administered intravenously is well tolerated in patients with normal renal function. In patients with renal compromise, care must be given when administering IV iodinated contrast to prevent additional renal insult. Over the last several decade, there has been a tremendous number of publications, policies, and guidelines describing the potential harm of iodinated contrast in the renally impaired and attempting to address safe contrast administration. Until recently, the risks associated with iodinated contrast have been mainly focused on intra-arterial administration of contrast (predominantly that administered for cardiac catheterization). Intra-arterial contrast administration is NOT the same as IV administration. The concentration of contrast imposed upon the kidneys is much higher and the volumes are usually much higher. Furthermore, catheter angiography also imposes the risk of microemboli to the kidneys, thus adding to the potential for renal insult–which is not the case for IV contrast. IV contrast is typically administered in volumes of less than 100mL and is much more dilute when it reaches the kidneys. Therefore, deriving risks of iodinated contrast administration from intra-arterial data and applying it to intravenous contrast administration exams is innappropriate and misleading.
Small studies reporting risks of IV contrast administration with respect to renal injury have been accumulating over the last decade or so. These studies have not demonstrated a significant risk to renal function in nearly all cases. Most recently, the Mayo Clinic reported a retrospective study of over 150,000 patients that were followed after CT examination and renal function changes were observed. In this study reported in Radiology April 2013, the authors reported that patients receiving contrast were not at an increased risk of developing further renal impairment after receiving contrast compared with those who had non-contrast CT examinations. This was the case even for patientes with serum creatinine levels of greater than 2.0 upon initial presentation (before receiving contrast). The authors raise the point of the possibility that IV iodinated contrast administration related acute kidney injury may be a misattribution. In other words, patients presenting to the hospital that may be seriously ill and receive contrast, and then develop renal failure would probably have developed renal failure regardless of contrast administration. For example, if a patient presents with mild renal compromise and severe sepsis receives contrast, their development of renal failure is NOT absolutely related to the contrast. Such patients have a high rate of developing acute kidney injury because they have severe sepsis.
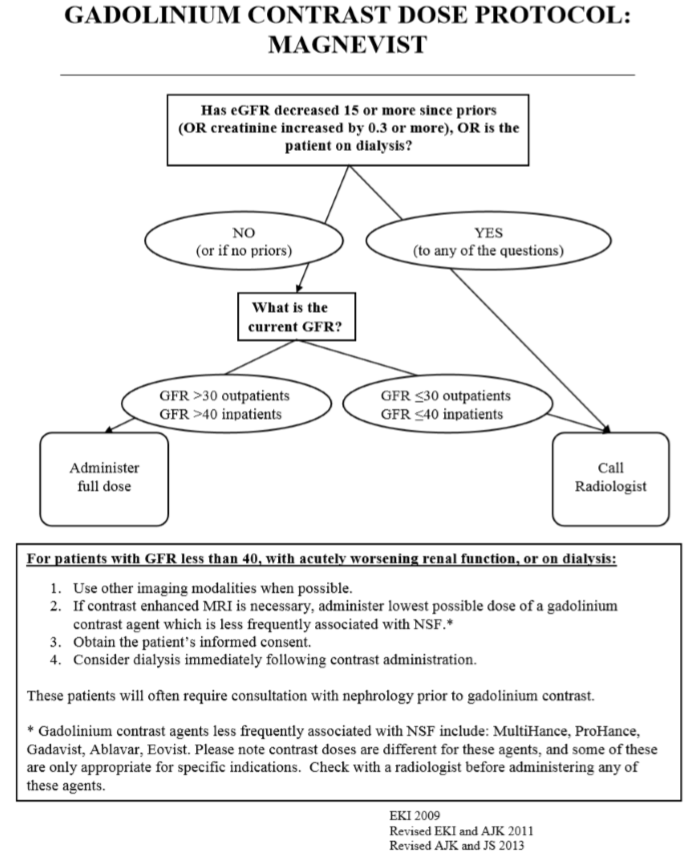
We have developed an evidence-based approach to administering IV iodinated contrast. We screen patients to determine if eGFR measurement is necessary prior to IV iodinated contrast administration based on risk factors that help predict renal impairment (e.g. age, diabetes, and personal history of renal impairment). Patients with moderately compromised renal function receive hydration before and after their examination, and the contrast dose is reduced. For patients with severe renal impairment (eGFR <31), contrast may be witheld, but even this is not an absolute contraindication. The risk-benefit ratio is contemplated in consultation between the radiologist and the patient's clinician to determine if contrast should be given (such patients will also be hydrated before and after contrast administration). We have established specific hydration guidelines to facilitate the process. The diagram below summarizes our approach. Note that patients with a recent decline in renal function are given special attention as such patients may pose a higher than average risk of contrast-induced nephropathy. 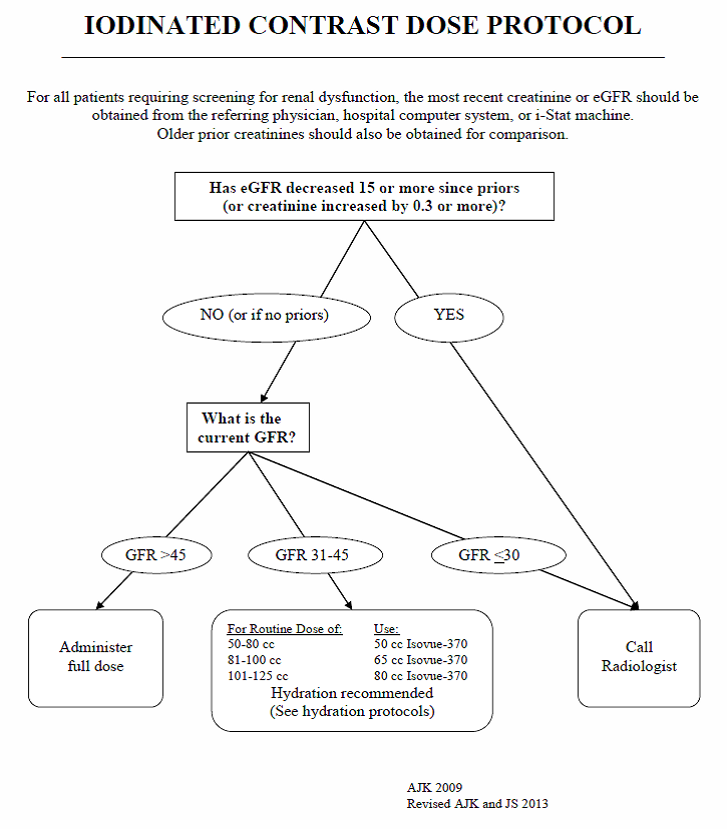
GADOLINIUM AND NEPHROGENIC SYSTEMIC SCLEROSIS
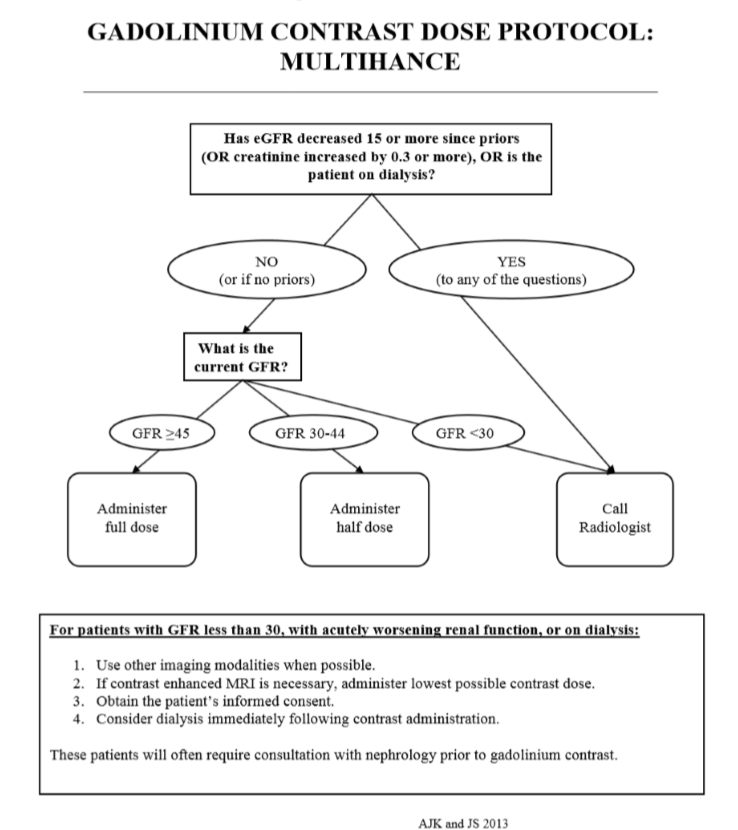
MULTIHANCE DOSE PROTOCOL
MAGNEVIST DOSE PROTOCOL